XIV. OXO PROCESS
Introduction:
The OXO Process consists of the reaction of olefins with water gas in the presence of Fischer Tropsch catalyst to give aldehydes according to the general equation.
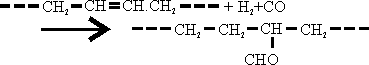
The aldehydes are hydrogenated to alcohols in a subsequent step.
The basic reaction was discovered by Ruhrchemie but the large-scale development of a continuous process resulted from a co-operative effort by Ruhrchemie and the I.G. The Chemo-Gesellschaft was a development organization formed by these two companies on a 50/50 participation basis. The process was later licensed to the operating company - the OXO Gesselschaft.
Extensive research on the process was carried out at Leuna where a plant of 100 T/month design capacity operated for 1½-2 years. The actual production was, however,only 40/50 T/month because of shortage of the preferred raw material -Kogasin. Leuna processed a certain amount of “Gelböl”, an olefinic by-product of the higher alcohols process to supplement their Kogasin supplies but it was stated that it was an inferior raw material. The present report is limited to information obtained at Leuna. Details of Ruhrchemie work are given in other reports, notably that on the Ruhrbenzin A.G. Target No. 30/5.01.
Chemistry of the OXO Process:
The first step in the process appears to consist of the addition of CO to the olefine according to the equation-
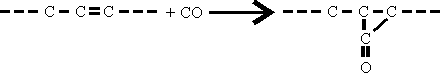
This intermediate product cannot be isolated because it is hydrogenated immediately to give aldehydes as follows:
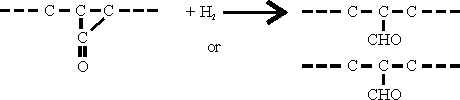
dependent on the point at which hydrogen enters to molecule.
Further hydrogenation, mainly carried out in a separate reaction stage, yields the corresponding primary alcohols.
It will be seen that oven in the simplest case, the OXO Process gives a mixture of aldehydes or alcohols. This tendency towards a mixed product is further increased by isomerisation of the olefine under the OXO Process conditions, thus:
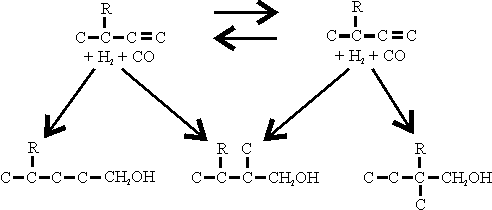
The reaction involving the least sterie hindrance predominates. Thus, using isobutylene as the olefine
| is obtained in greater quantity than | 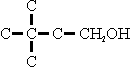 |
Similarly, when using trimethylpentenes obtained from polymerisation of
iso-butylene, the main OXO products are those derived from the isomer as
distinct from the 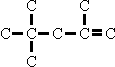 isomer as distinct from
isomer as distinct from 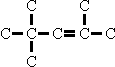
The mixed alcohols obtained from the OXO Process are mainly used after suphonation for detergent manufacture. For this purpose the mixed alcohols are said to be better raw materials than single compounds.
Process Conditions:
The first stage of the process is carried out at about 200 ats. pressure and 150°-160°C in the liquid phase. Finely divided Fischer Tropsch catalyst is suspended in the liquid feed in a concentration of 3-5% by weight. Most of the catalyst is recycled and the make up requirement is said to be very small. Normally, when using Kogasin as the olefinic feed, the reaction time required is of the order of 20 minutes is of the order of 20 minutes. Lower olefins reacted very readily. In the case of low molecular weight olefins, they have to be used in solution in a liquid medium.
A number of side reactions occur in the OXO stage. Aldehydes polymerize to give “Dicköl” which comprises up to 20% of the crude product. About one third of the aldehydes initially formed are also hydrogenated to the corresponding alcohol. It is thus not practicable to isolate aldehydes from the crude product obtained in the first stage of the process. If these products are required, it is considered preferable to complete the hydrogenation in the second step, separate the alcohols and oxidize them to the corresponding aldehydes.
This second step of hydrogenation in addition to converting the aldehydes in the alcohols, breaks down about 50% of the Dicköl to alcohols of the same composition as are derived from the corresponding aldehydes. The hydrogenation is hindered by the presence of CO. It is therefore necessary to let down to atmospheric pressure the crude product from the first OXO stage and to carry out the hydrogenation in a separate step. This stage is carried out at about 200 ats and at a temperature of 170-195° C. The same catalyst can be filtered out of the first stage crude and it can be replaced by the more readily available copper chromite. When Fischer Tropsch catalyst is employed, some carbon monoxide is formed in the hydrogenation reaction as a result of reduction of cobalt carbonyl. In order to keep down the concentration of carbon monoxide in the circulating hydrogen, the exit gas is treated over an iron catalyst to convert carbon monoxide to methane. The methane content of recycle gas can be as high as 10% without adverse effect on the reaction. This concentration is maintained by bleeding off the requisite amount of gas from the circulating system.
The first step of the OXO Process is not affected by the presence of sulphur compounds in the raw materials but these impurities do hinder the chromite catalyst. When dealing with sulphur-containing olefine raw material, therefore, it is necessary to filter off the first-stage catalyst and to carry out the hydrogenation over fixed nickel tungsten sulphide catalyst. It is necessary to carry out a partial hydrogenation of the crude first-stage product prior to filtration in order to convert any cobalt carbonyl into cobalt.
Leuna Operation:
The process, as originally worked out by Ruhrchemie, was a batch process and the pilot plant at Holten consisted of 18 units originally intended for batch operation. Work at Leuna showed that considerably higher through-puts were obtained from continuous operations.
The following description of the latest method of operation of the Leuna plant was obtained by W.A. Horne from Dr. Gemassmer, who was the chemist directly in charge of these operations. It should be read in conjunction with Fig. XXX.
The olefine or olefine-containing charge is mixed with 3-5% by weight of catalyst, most of which is recycled material. This suspension is pumped at the rate of 300-700 litres/hour and at a pressure of 220-240 ats. through a heater which raises its temperature to approximately 150°C. The preheated feed enters the bottom of the first reactor and passes upward concurrently with a steam of 60 M3/hour of carbon monoxide and hydrogen which has been separately preheated to 150-190° C (maximum: 200°C). This synthesis gas is partly recycle gas from the process (40-50 M3/hour) and partly make up gas which consists of equal molecular proportions of hydrogen and carbon monoxide. The first reactor, which is constructed of carbon steel, has an internal diameter of 200 mm. and a length of 8 M. It contains 6 vertical 21 mm. OD, 17 mm. ID, steel cooling tubes which are connected to a water jacket surrounding the reactor. Cooling by these tubes is used only when very reactive olefins are charged and the heat release is high. A thermocouple well extends the length of the reactor and the temperature of the exit products is normally controlled at 150°C. The temperature and feed rate depend on the concentration and molecular weight of the olefins in the charge stock. As previously stated, lower molecular weight olefins are more reactive. Low concentration of olefins in the feed necessitates the use of lower feed rates and higher temperatures in order to ensure that reaction proceeds to the required extent. Normally, roughly 70% of the olefins charged are converted in the first reactor.
The exit products from the top of this first reactor pass to the bottom of the second reaction vessel where they come into contact with an additional 60 M3/hour of synthesis gas. The second reactor has the same dimensions as the first by is fitted with baffles to increase the efficiency of contact. No cooling tubes are required. The normal operating temperature is 170° C. Essentially all the remaining olefins are converted and some 20% of the aldehydes made are hydrogenated to alcohols.
The exit products from the top of the second OXO reactor now flow through a water cooler to a separator from which synthesis gas is recycled to the preheater. The liquid product is let down to atmospheric pressure and the released dissolved gases are purged after scrubbing with crude second-stage product to prevent loss of liquid by entrainment. The first-stage product is now pumped under a pressure of 200-250 ats. to the second-stage preheater from which it passes to the bottom of the first reactor of the hydrogenation stage. 60 M3/hour of a mixture of preheated fresh hydrogen and methanised recycle gas is also introduced at the bottom of the reactor. The reactor is identical with the first reactor of the OXO stage by operates at an exit temperature of this second reactor is roughly 200°C. The draw-off of liquid product from the bottom of this converter is regulated so as to keep the reactor full of liquid. The top of the second reactor serves as a high pressure separator vessel for hydrogen and liquid products. The hydrogen containing some carbon monoxide is water-cooled and passes to a catch-pot for separation of condensed liquid which is returned to the hydrogenation reactor. Before being recycled to the hydrogenation reactors, the gas is reacted at 250° C over an iron catalyst (similar to that used in the Synol Process) in order to convert carbon monoxide to methane.
The liquid product is let down to a pressure of 10 ats. to a separator from which dissolved gases are vented. The liquid from this separator is charged under its own pressure to the filter system illustrated in Fig. XXXI. The liquid, in batches of 700 litres, enters the filter vessel (which is pressured with nitrogen through Valve 1) and is filtered through the porous ceramic tubes situated at the bottom of the vessel. This operation requires about 10 minutes with a new filter but can take up to 30 minutes when the filters are old. When filtration is complete, fresh olefine charge is introduced through Valve 3 and passes in the reverse direction through the filter thereby washing off the catalyst material. The whole vessel is rotated at 60 revs/minute for 2-3 minutes. It is stopped in the inverted position and the olefine catalyst suspension is forced out through Valve 4 by nitrogen pressure, nitrogen being introduced through Valve 3. This suspension is then transferred to the feed mixer of the OXO Process. The cycle time for a complete operation of the filter is one hour per batch of 700 litres of crude product.
The treatment of the filtrated crude product depends on the type of olefinic raw material used. If this raw material has initially a boiling range not exceeding 30°C, the alcohols can be separated from hydrocarbons by simple distillation. If, on the other hand, a raw material of wider boiling range is employed, alcohols have to be separated by the boric acid method as described in Section XV dealing with the Synol Process.
A number of variations of the above process had been tried out at Leuna. The effect of introduction of liquid feed at the top of one or both of the OXO reactors was tried, as was also the operation of the OXO Process with liquid and gas flowing counter-currently. The process was also operated with only one reactor in the OXO and hydrogenation stages. According to Dr. Gemassmer, however, the method described in detail above was found to be the most satisfactory.
An essential of any scheme for operating the OXO process is that the synthesis gas rate in both the OXO and hydrogenation stage must be sufficiently high to ensure efficient stirring and complete suspension of the catalyst . A large excess of synthesis gas is not necessary for the purely chemical standpoint. Research carried out by the I.G. suggests that the OXO stage might be operated at 40-50 ats. pressure but under these conditions the throughput would be lower and the temperature somewhat higher. One of the difficulties sometimes encountered was that unless the conditions in the OXO stage are carefully controlled olefine polymerization takes place. The polymers so formed, after hydrogenation in the second step, are difficult to separate from the higher boiling alcohol products.
Catalyst Preparation:
The Fischer Tropsch catalyst used in the OXO Process was obtained from the catalyst plant of Ruhrchemie at Oberhausen-Holten. It was reported to have the following approximate composition:
30 % Cobalt
2% Thorium oxide
2% Magnesium Oxide
66% Kieselguhr
Due to the scarcity of cobalt, the content of this component of the catalyst has latterly been decreased. The last shipment contained only about 25% of cobalt. This apparently had little effect on the process operation.
The catalyst in powder form is reduced with pure sulphur-free hydrogen. the hydrogen flow is controlled at a rate high enough to prevent settling of the catalyst powder, i.e. the catalyst is fluidized by the hydrogen stream. The period of reduction is 2-4 hours.
OXO Processing of Cracked Middle Oil:
In addition to Kogasin and Gelböl, Leuna had investigated the treatment of cracked petroleum oil by the OXO Process. Dr. Gemassmer provided the following date from a run, the conditions of which were not considered to be optimum.
The olefinic feed material had the following properties:
|
Density |
0.848 |
|
Pour Point |
-18° C |
|
Av. Molecular Wt. |
195 |
|
iodine No. (Hanus) |
46-48 |
|
Sulphur Content |
.24% by wt |
|
Boiling Range |
230°-350° C |
|
Volume % soluble Kattwinkel solution |
51. |
The charge was mixed with 3 % by weight of catalyst and reacted with water gas at 240 ats. Total reaction time in the OXO stage was approximately 1 hour. The temperature at the inlet to the first reactor was 150 C and that at the exit of the second reactor 190 C. The hydrogenation of the crude product was carried out at 220 ats with hydrogen of 97% purity. The temperature was 195-200 C and the total reaction time approximately 1 hour. The product had the following properties:
|
Density |
.862 |
|
Iodine No. (Hanus) |
19.6 |
|
OH No. |
36-40mgm KOH/gm |
|
CO No. |
3.5 |
|
Saponification No. |
5 mgm KOH/gm. |
The OH number is determined by acetylation with acetic anhydride followed by titration with KOH solution. The CO umber is a measure of the aldehyde and ketone content and is determined by forming the oximes of aldehydes and detones followed by titration with KOH solution.
The crude product contains 16-17% alcohols which may be separated from the hydrocarbons by forming boric esters. It is not possible to separate the alcohols by simple distillation because of the wide boiling range of the charge stock.
Only about 60% of the olefins was converted and of this, approximately 80% was recovered as alcohol.
Fig. XXXI (B) is a photograph of the OXO converters.
In addition to the authors, Major A.V.J. Underwood contributed notes and these were of very great assistance in compiling the above report.