SECTION I. (b)
(1) Fe Catalysts-General. Historically, Fe catalysts are older than cobalt. The first synthesis was carried out in 1922 over Fe catalysts. These tests were conducted at high pressures (100 atm.) and temperatures (400°C). The results were exclusively oxygenated compounds. With decreasing pressure the oxygenated fractions of the products decreased until at around 7 atm., the yield was almost exclusively hydrocarbons.
The decrease in pressure resulted in a corresponding drop in reaction velocity. It was therefore necessary to develop more active catalysts. At the temperatures employed at the time (400° C) these active catalysts. Cobalt was found to fulfill the requirement and iron was temporarily shelved.
Nevertheless, work on iron catalyst was continued because the lower cost and higher production of olefins were were obvious advantages. The main difficulty was the low activity forcing the operation into a temperature range where CO decomposition (carbon deposit) may occur and where the lighter members of the paraffin homologues are more likely to be found. (This conversion at higher temperature under otherwise equal conditions yields more methan and gasol.)
Before going into some of the details of the German development of iron catalysts it seems appropriate to consider the effect which the elevation of the temperature range has on the equilibrium of the FT reaction. (See reference I(b)1 and 1(b)2 at end of this section). At first it can be shown that lighter products may be expected at higher temperature, other conditions being equal.
The following table gives values of the equilibrium constant “K” for the Fischer-Tropsch reaction for different temperatures and different members of the hydrocarbon series.
The values were calculated by P. Dolch, for the equation:
![]()
![]()
![]()
![]()
![]()
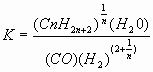
|
Compound |
CH4 |
C2H6 |
C3H8 |
C4H10 |
C6H14 |
C8H18 |
|
Temperature |
||||||
|
100° C |
-17.69 |
-13.56 |
-12.17 |
-11.67 |
-10.77 |
-10.44 |
|
200° C |
-11.32 |
-7.93 |
-6.85 |
-5.53 |
-5.85 |
-5.49 |
|
300° C |
-7.15 |
-4.27 |
-3.38 |
-3.18 |
-2.50 |
-2.26 |
|
400° C |
-4.23 |
-1.70 |
-0.94 |
-0.83 |
-0.19 |
+-0.00 |
Note: That the equilibrium is favored by lower temperature and that the lower boiling hydrocarbons are more likely to be formed at higher temperature.
Another fact which should not be overlooked, is the effect of feed gas composition on the equilibrium. This effect is much greater at the higher temperature and may afford a means to direct the synthesis more efficiently.
The data below are shown to indicate the effect of temperature on the susceptibility to changes in feed gas competition. The figures are % yield, based on CO fed for the reaction with n=8 (Octane) at equilibrium, 1 atm. And 349° C.
| Feed gas: | Equivalent 1:2 (CO:H2) “ideal” | 68.3% |
| 13.8% N2 Inert | 62.9 | |
| Steam: ½ part/1 part CO | 54.7 | |
| Steam: 1 part/1part CO | 41.6 | |
| *Excess hydrogen: 5.9% | 71.0 | |
| *Excess Carbon monoxide: 17-7% | 57.7% | |
|
*(Over the ratio 1:2 CO:H2) |
On the other hand the corresponding figures for 180° C and butane as the product vary only between 99.42 and 99.69%. The results of the operation depend on many other conditions besides the equilibrium, as shown above, but the effect of temperature in this connection is quite evident.
The following sections report in condensed from the work done by German research on the development of iron catalysts.