7. Separation of Product.
The separation of the various products has been the subject of considerable work by I.G.
(a) Pretreatment
Before the alcohols, olefins and the neutral oil can be separated, it is necessary to take certain steps regarding the undesirable by-products. As such, are classified the acids and their reaction product with alcohols, the esters. Other undesirable compounds are the aldehydes and ketones which appear in only small quantities.
The acids must be neutralized with alkali to prevent further alcohol losses to esterification. A simple caustic wash is sufficient for this purpose. If it is also desired to de-esterify the product, the caustic wash may at first be omitted.
The esters are preferably saponified with strong alkali at 130° C. A 30 minutes treatment with good agitation is sufficient. The alcohols are thereby set free and the acid neutralized y the alkali. At the same time the reactive aldehydes are condensed to form heavy polymers and thus wind up in the high boiling fraction (se on the next page).
If the esters are not removed, they appear in the following fractionation according to their boiling range and thus induce alcohols and acids into these fraction where they do not belong.
(b) Fractionation. (See reference II/21 at end of this section).
A sharp fractionation of the raw product is apparently essential for the exact separation into alcohols and hydrocarbons. This is particularly the case when boric acid-esterification is used as a means of separation.
The lower alcohols (to C3) are practically completely dissolved in the aqueous phase of the product. The C4 to C6 alcohols may be washed out with water or possible methanol-water solution. This water wash is automatically carried out when the product is first de-esterified since the alkali must be washed out with water.
The higher alcohols may be separated and prepared in 95-97% purity, provided the original feed is fractionated sharply. Several methods were studied by the Germans.
A simplified flowchart of a synol process may look as follows:
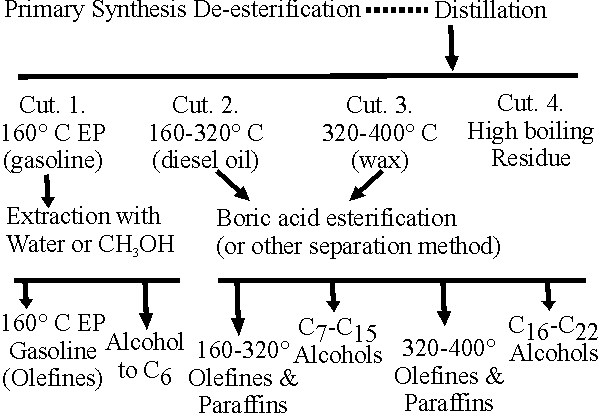
(c) Boric Process. (See reference II/22 at end of this section).
Boric acid esters are used for the separation of higher synol alcohols from the accompanying olefins and neutral oil. It is essential that the feed to the esterification unit be fractionated as sharp as possible 50° C boiling range appears to be satisfactory for each cut.
One of the difficulties which require further work is the distillation of the neutral oil from the boric acid ester. The mixture must be thoroughly agitated. At the same a temperature in excess of 250° C must be avoided in any step of the process.
The highest alcohol produced to date by this method is C22 H45OH. Higher alcohols cannot be separated from the oil without decomposition.
The principle of the process is based on the fact that:
In the process of this type the boric acid used for esterification must be substantially pure B2O3. But at the same time only a dilute solution of B2O3 is obtained in hydrolyzing the ester. This operation is expensive since large quantitites of solution must be concentrated.
It was found however, that a saturated aqueous solution could be separated from the free alcohol by settling, and upon cooling, the additional free acid would precipitate from the solution. The acid crystals could be recovered by filtration and used immediately for further esterification. The process is applicable to all types of alcohols and in particular for the oxo process.
The esterification yields a certain quantity of water which must be removed. This is done by extractive distillation with benzine. The esterification requires elevated temperature, and thus the water and benzene are simply carried overhead as they form.
Following the removal of the water, the neutral oil is distilled to leave pure ester in the kettle of the column. The ester is then hydrolyzed with hot aqueous boric acid solution.
Below find operating data for one example:
|
The raw material from the synthesis has the following composition: |
||
| Alcohols |
50% vol. |
|
| Olefines |
40% vol. |
|
| Paraffins |
9% vol. |
|
| Acid, Ketones etc. |
1% vol. |
|
|
100% vol. |
||
|
This mixture was fractionated to give the following cuts: |
|||
|
(1) |
0-100° C |
30% vol. |
|
|
(2) |
100-200° C |
20% vol |
|
|
(3) |
200-300° C |
16% vol |
|
|
(4) |
300-400° C |
17% vol |
|
|
(5) |
400° C+ |
17% vol |
|
|
100% vol |
|||
The object was to recover the alcohols boiling between 200° and 400° C. For this purpose fraction 3 and 4 were treated separately.
| Treatment of fraction 4: | |
| Feed: | 100 kg. of fraction containing 72% wt. alcohol. |
| 6.6 kg. boric acid. | |
| 575 kg. benzene | |
| The mixture is agitated and heated to 90° C for 2 hours. | ||
| 575 kg. benzene | }Distilled overhead | |
| 4.8 kg. water | ||
| Distillation is then continued under vacuum. | ||
The neutral hydrocarbons distill overhead. A total of 28 kg. is recovered in the receiver.
The kettle product, i.e., the boric acid ester after is transferred to the saponifier, where it is treated at 95° C for 30 minutes with 55.5 kg. of a 2.6% aqueous boric acid solution. The free acid solution is then allowed to settle at 95° C and is withdrawn. The solution now containing 10.9% wt. (i.e., 38.8% C) is next cooled to 2° C. The excess boric acid crystallizes and is centrifuged. 5.2 kg. boric acid are thus recovered. The filtrate, 50.5 kg., is returned to the system.
The free alcohols, 72 kg., are also withdrawn from the saponifier. 1.5 kg. boric acid still remain in solution. For its removal the alcohols are washed with 56.2 kg. water. The alcohol passes 3 counter current was stages.
(d) Separation by Silicagel Adsorption. (see ref. II/25 at end of this section).
This method was considered by the Germans at one time for the separation of synol alcohols and deserves to be mentioned here.
The method is based on the difference between the heat of adsorption between alcohols and hydrocarbons.
|
Heat of Adsorption |
|
|
methanol |
15.0 Cal/gm Silicagel |
|
ethanol |
14.8 Cal/gm Silicagel |
|
n + butanol |
13.0 Cal/gm Silicagel |
|
n + octanol |
12.0 Cal/gm Silicagel |
|
n + decylalcohol |
12.7 Cal/gm Silicagel |
|
n + hexane |
5.3 Cal/gm Silicagel |
|
n + dodecane |
6.1 Cal/gm Silicagel |
|
water |
16.1 Cal/gm Silicagel |
The mixture is contacted with the dry gel and then washed with a volatile low boiling hydrocarbon solvent (petrol ether). The hydrocarbons are thereby removed. The alcohols remain adsorbed and may then be removed by such polar solvents as lower boiling alcohols or ketones and ethers. The gel is finally regenerated.
The gel may be loaded according to its quality, the particle size (1-2 mm.) and the alcohol boiling range. For 170-180° alcohols, 15 g/100 g gel may be reached.
The adsorption must be carried out at room temperature or even below. The alcohol should be desorbed as soon as possible. The regeneration of the gel may be carried out with hot gases (N2, CO2) at 150° C and 1:1000 V/H/V.
(e) Separation of Azeotropic Distillation. (see ref. II/24 at end of this section).
Glycols are known to form azeotrope mixtures with many classes of organic compounds. In the case of synol products it was found that glycols may be used for separation because:
Particularly suited are of course those glycols which form azeotropes with hydrocarbons only and not with the alcohols. Butanediol 1-3 and hexane-diol 1-6 fall in this category.
Another particular advantage of these compounds is their high efficiency, i.e., the low volume ratio between the azeotropic carrier and the overhead component. This ratio may further be lowered b y operation under vacuum. The following figures may serve as an example:
Using butanediol 1-3 on a 230-245° fraction the ratio of glycol to hydrocarbon is 2:1 at 1 atm., but only 1:1 at 20 mm. Hg.
In selecting the third component it is desirable to have the initial boiling point of the mixture 10-20° C above the boiling point of the carrier. The other oxygenated compounds such as ketones and esters are usually distilled overhead with the hydrocarbon.
It is possible first to enrich the mixtures of synol products in alcohol content by selective extraction with methanol. Thus 80% alcohols may be obtained in the mixture. 100 pts. of such a mixture (230-270° C) are distilled with 10 parts butanediol 1-3 at 20 mm. Hg. to give an alcohol concentration in the kettle as high as 98%.
The process is generally limited to alcohols from C8 on up.
(f) Separation by Extraction with Aqueous Methanol. (See ref. II/23 at the end of this section).
The process is based on the higher solubility of the alcohols in the methanol solution. The necessary quantities to reach an alcohol concentration of 97% are given below.
|
Synol Fraction |
Alcohol Content |
CH3OH Conc’n used |
Vol. CH3OH Solution for 1 vol. synol |
% Alcohol in the neutral oil |
% Non-Alcohol in the Alcohol |
|
120-170°C |
56 |
68 |
2 |
0.1 |
3.0 |
|
170-220°C |
48 |
76 |
3 |
0.3 |
3.0 |
|
220-270°C |
40 |
81 |
6 |
0.6 |
3.5 |
|
270-320°C |
29 |
85 |
10 |
1.0 |
3.5 |
For practical purposed the extraction method is limited to C12 alcohols maximum.
The methanol is separated from the extract by distillation. Upon removal of the CH3OH, the higher alcohols and the water separate into two phases and the water is withdrawn.
The methanol extraction or enrichment may be of interest in combination with the use of glycol to obtain final alcohol concentration (see above).
(g) High Boiling Products.
It was pointed out that prior to separating the alcohols from the neutral oil, the raw synol product had to be fractionated. The fraction boiling above 400°C was not considered in the process. Alcohols above C22 cannot be prepared by the boric acid method as the neutral oils cannot be removed from the ester by distillation.
The high boiling product is dark brown, probably due to Fe which is contained in quantities up to 0.1% (due to iron carbonyl). The product may be used as such for certain industrial purposes. it can be treated with Fullers earth to give slightly yellow waxes. Pour points are usually varied from 70 to 105° C. The high ester content of these products makes them more ductile than FT wax. If necessary they may be hydrogenated to give high melting point waxes of the FT type.