THE SYNTHESIS OF HYDROCARBONS AND CHEMICALS FROM CO AND H2
SECTION V
ISOBUTANOL SYNTHESIS
SUMMARY
The attached report covers information regarding the synthesis of isobutanol and higher alcohols by a modified h.p. methanol process.
The isobutanol synthesis was a prewar I.G. development and was practiced extensively for the production of isobutylenepolymer (appanol) and iso-octane. The high boiling fractions, while only a small percentage of the total product were of great interest for the production of detergents and lube oil esters.
1. Isobutanol Synthesis.
The following information on the synthesis of isobutanol was obtained from Dr. Goggel of the I.G. Farbenindustrie at the Ludwigshafen plant on 28 May 1943. This synthesis is in essence an extension of the high pressure methanol synthesis which utilizes carbon monoxide and hydrogen. The isobutanol synthesis uses the same raw materials, practically the same catalyst, and pressures of the same magnitude (about 240 atmospheres). The catalyst used for the higher alcohols in zinc and chromium oxides with the addition of one percent of potassium hydroxide. The temperature used is about 430° Centigrade.
The main difference compared to the methanol synthesis is the lower output per catalyst volume, since the main product (CH3OH) is recycled to extinction. Based on a once-through operation, the product made in greatest quantity in this synthesis is methanol; about five to six parts of methanol are obtained for every part of isobutanol. In addition to this, there are a great many other alcohols and ketones produced. The total weight of these products equals the weight of isobutanol in the product.
| On a water free basis the total product contains approximately: | |
| Isobutanol | 14% |
| Methanol | 63% |
| High alcohols and Ketones | 15% |
| Hydrocarbons | 5% |
| a. | 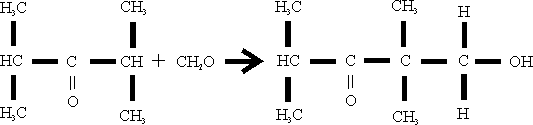 |
| b. | 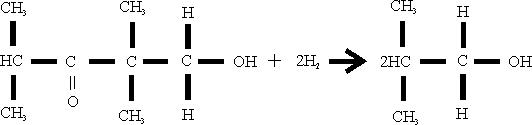 |
(See also reference V/6 at the end of this section).
The separation of the products from this synthesis is by fractional distillation. The equipment involved is not novel in any of its features but is complex only because of the multi-component mixture which must be separated. The distillation scheme is straight forward, using continuous flow through columns which remover one component at a time. (see ref. V/1 to V/5 at the end of this report).
2. Details.
In the isobutanol synthesis a feed gas of approximately the following composition is used:
|
Carbon monoxide |
32% |
|
Hydrogen |
57% |
|
Nitrogen |
balance |
The catalyst is a pelleted catalyst with no binder, composed of the following:
|
Zinc oxide |
60 parts |
|
Chromium oxide |
40 parts |
|
Potassium hydroxide |
1 part |
The zinc oxide is broken up and chromic acid is added. The mixture is worked ¼ hour, then 0.5% graphite is added as lubricant. the mixture is then moistened with distilled water (100 kg. ZnO, 60 kg. Cr2O3, 20 lit. H2O). A reaction occurs in ¼ hour. The catalyst is then pressed into pellets 5-5 mm. 1% KOH is added to the final catalyst in form of fine K2CO3 powder. This is the only difference between isobutyl and methanol catalyst. The catalyst is charged to the chamber and reduced in the place. The conditions for the reaction are:
| Pressure | 240 atmospheres |
| Temperature | 430° Centigrade |
The composition of the gas leaving the converter is approximately as follows:
|
Carbon monoxide |
22% |
|
Carbon dioxide |
5-6% |
|
Hydrogen |
57% |
|
Nitrogen |
5% |
|
Hydrocarbons (mostly CH4) |
Balance |
The analysis of the liquid product is approximately as follows:
|
Isobutanol |
12% |
|
Methanol |
55% |
|
Water |
18-20% |
|
High alcohols and Ketones |
10% |
|
Hydrocarbons |
Balance |
All methanol is recycled to the chamber. It is impure and purification cannot be economically justified. The recycle has no effect on the isobutanol yield. The methanol in the feed is considered simply like CO+2H2. A detailed listing of the compounds contained in the product is given here to illustrate the complexity of the composition. These are taken from the attached chart.
| (a) | Hydrocarbons, total about 5 percent. | ||
| Propylene | Diisoblutylene | ||
| Propylcylohexane | Triisobutylene | ||
| (b) | Alcohols - total about 70-75% | ||
|
Methanol |
Propanol | ||
| Ethanol | Butanol | ||
| n-amylalcohol | Isobutanol | ||
| Ethylisopropylcarbinol | Diisopropylcarbinol | ||
| Dimethylclohexanol | Sec.-butylcarbinol | ||
| Aldehydes - total about one per cent | |||
| Formaldehyde | |||
| Isobutyraldehyde | |||
| Diethylacetaldehyde | |||
| Ketones - total about 8-10% | |||
| Acetone | |||
|
Methylethylketone |
|||
| Methylpropylketone | |||
| Ethylisopropylketone | |||
| “Isobutyron” (Diisopropylketone) | |||
| etc. | |||
| The balance if made up of acids, aletones, and phenols | |||
The condensation “isobutyron” with formaldehyde is as follow:
The materials are utilized in these ratios:
|
Material |
Mol.. |
|
500 tons per month of 90% Isobutyron |
3.95 |
|
500 tons per month of 30% Formalin |
5.00 |
|
130 tons per month of 20% Sodium Hydroxide |
0.65 |
|
500 tons per month of methanol (Solvent) |
These are charged batchwise to agitated autoclaves in such a manner that the sodium hydroxide catalyst is added over a period of 9 hours, while the temperature is maintained at 50° C/ At the end of this period the crude condensation product is charged to a still in which the methanol is removed. The residue product from this distillation is washed with water to remove salts and the remaining formaldehyde, and is neutralized by the addition of sodium sulphide The washed crude product is then distilled to separate the unreacted “isobutyron” from the condensation methylol-isbutyron.
The isolated methylol “isobutyron: is then hydrogenated over a copper chromite catalyst at 200 atmospheres and a temperature of 180 to 200°C.
The product is purified by a fractional distillation in which methanol is removed from the top of the column, pure isobutanol is taken off as side steam, and the high boiling residues are taken off at the sump.
About 400 tons per month of isobutanol are obtained from 500 tons of “isbutyron:. This is a yield of about 62% of the theoretical.
The isobutylalcohol is dehydrated over alumina to isobutylene at 330-360° C. The best temperature gives 95% conversion and the operation is once through. No recycle is required. The alumina has a 3-4 month life.
Isobutylene was used for iso-octane production and as feed stock for oppanol (polyisobutylene).
While the isobutysynthesis was of importance during the war for the production of iso-octane, it was claimed that the process was equally of peacetime value as a producer of isobutylenes for appanol and particularly for the higher alcohols boiling from 180 to 250° C. This fraction was used for the production of the “Zornol” (synthetic lubricants).
3. List of References.